- Home
>
- Water Story
>
- What is Electrolyzed Water?
What is Electrolyzed Water?
Definition of Oxidized Electrolyzed Water
While residential alkaline electrolyzed water is water for drinking and skin cleaning purposes
that is electrolyzed by passing a weak current by adding calcium glycerinate to water that has
been chlorinated with activated carbon, strongly acidic water is water for sterilization and disinfection
purposes that is obtained by electrolyzing water by adding NaCl (table salt) or Kcl (potassium chloride)
with a purity of 99.0% or more to the anode tube of the electrode.
Characteristics
| Classification |
Tap water (groundwater) |
Oxidized electrolyzed water (strongly acidic water) |
Reduced electrolyzed water (strongly alkaline water) |
| pH |
6.8 ~ 7.7 |
2.4 ~ 2.7 |
11 ~ 12 |
| Hypochlorous acid (ppM) |
0.3 |
10 ~ 20 |
0.4 |
| Oxidation-reduction potential (ORP) |
350 ~ 350mV |
1100 ± 20mV |
-700mV 이상 |
| Dissolved Oxygen (ppm) |
9.2 |
25 |
- |
| O-NMR Half-Width |
140 ~ 150Hz |
55 ~ 62Hz |
55 ~ 65Hz |
| Conductivity (ms/cm) |
245 |
1,980 |
2,630 |
How it works
- Using a special ionic membrane that allows only ions with electrical properties to pass through
- In the anode chamber
· A reaction occurs in which negatively charged ions (OH-, Cl-, etc.) lose electrons (e-) to the electrode.
· Chlorine gas (C12) and hypochlorous acid (HClO) are produced.
· Some of the O2 gives e- to the + electrode and reacts with water to produce O3 (ozone).
· The decrease in OH- ions and increase in H+ ions results in a decrease in pH.
· This is represented by the chemical reaction equation
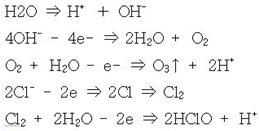
- In a cathode chamber
·
Positive (+) electrically charged ions (CH+) receive electrons from the electrode to produce hydrogen gas (H2).
· The pH rises due to the decrease in H+ ions and increase in OH- ions.
· The ORP drops due to the increase in electrons (e-).
· This is represented as a chemical reaction,